The size threshold for sexual reproduction
During size reduction, cells change in their physiology as well as their morphology. The best documented change is associated with sexualization. Within a particular species, the larger cells are incapable of sexual reproduction, no matter how they are treated. Smaller cells, on the other hand, can become sexualized if certain other conditions are met, such as particular nutrient concentrations, light regimes, temperatures, etc, or particular changes in these environmental parameters. For example, Lithodesmium undulatum becomes sexualized if smaller cells are transferred to higher temperatures (15 to 24ºC), brighter light, and new medium (Manton & von Stosch 1966; see also Drebes 1977). In most cases, we know very little about the environmental triggers for sexual reproduction. In Sellaphora, sexual reproduction occurs in a wide variety of laboratory conditions, providing a compatible mate is present.
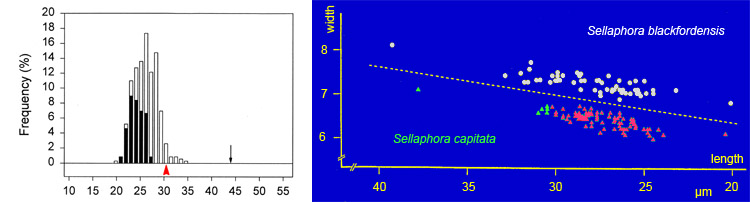
Thus, small cell-size is necessary but not sufficient for sexualization in diatoms. The transition from recalcitrance (inability to respond to sex cues) to potential compliance appears to be quite rapid – perhaps only a few cell divisions – so that it is is possible to distinguish a 'sexual size threshold' for each clone or population, which is a permissive threshold. The threshold for Sellaphora capitata seems to be just over 30 µm. Above the threshold, no sexual reproduction occurs (cells represented by green triangles in the figure); below it, sexual reproduction will occur if the cells (those represented by red triangles) are in the right environment. The size threshold for S. blackfordensis is higher – 36 µm.
Although the sexual size threshold seems to be abrupt within a clone, there is probably variation in the position of the threshold among different clones and populations of the same species. There may also be changes in the intensity of the sexual response as cells progress further below the threshold. Either or both could explain the left-hand graph shown above. In this (an analysis of a sexualized population of S. capitata), the vertical bars show the percentage frequencies of cells of different sizes, classified into those that were sexual (black) and those that remained vegetative (white). The total number of cells measured was 346. The proportion of cells sexualized rises from zero (at sizes of above, at and just below the maximum size for sex in S. capitata: red arrowhead), to near 100% in cells close to the minimum size seen in most natural populations (c. 20 µm).
The black arrow indicates the maximum size attained by S. capitata, through expansion of the auxospores after sexual reproduction.
The data for the graphs were obtained from Blackford Pond, Edinburgh. They are modified from Mann (1988) and Mann et al. (1999). Mann et al. (2004) give data on the sexual size thresholds of several Sellaphora species.

 This site is hosted by the Royal Botanic
Garden Edinburgh.
This site is hosted by the Royal Botanic
Garden Edinburgh.